Vaseghi Research Lab
The Vaseghi Lab
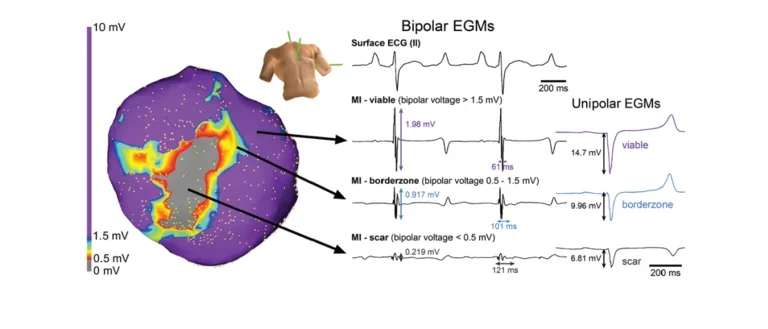
Bipolar EGMs
In-vivo electroanatomic map of a chronically infarcted porcine heart displaying evidence of low voltage and fractionated bipolar electrograms.
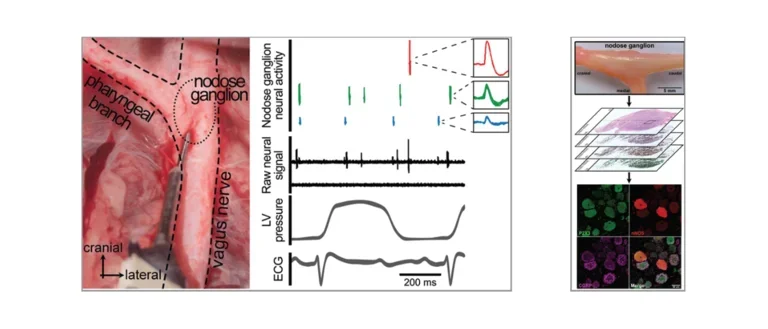
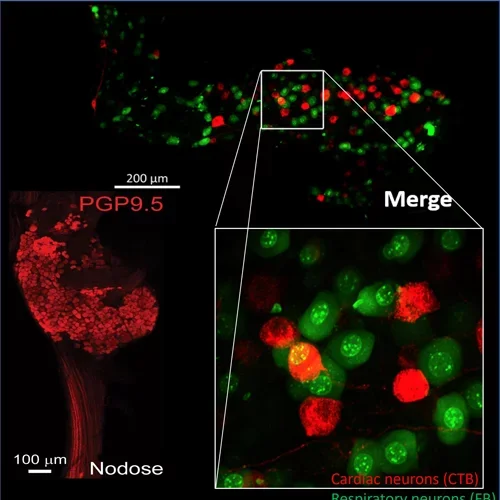
Nodose Ganglion
Left: In-vivo neural recordings from the porcine nodose (vagal) ganglion to assess changes in parasympathetic afferent transduction after myocardial infarction. Right: Histological evaluation of porcine nodose ganglion to assess diversity of neurons and changes in neuronal subpopulation with cardiovascular disease.
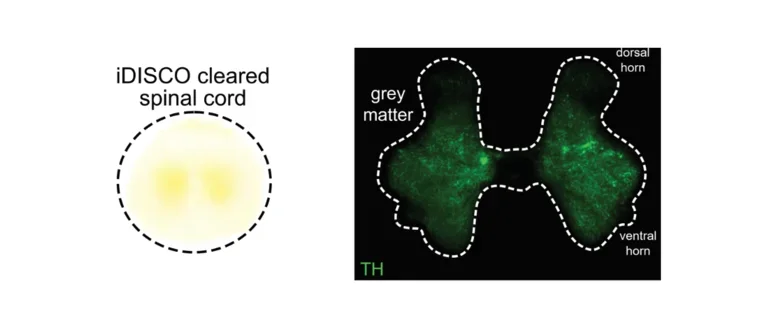
Spinal Cord
Tissue clearing and histology of a porcine spinal cord.
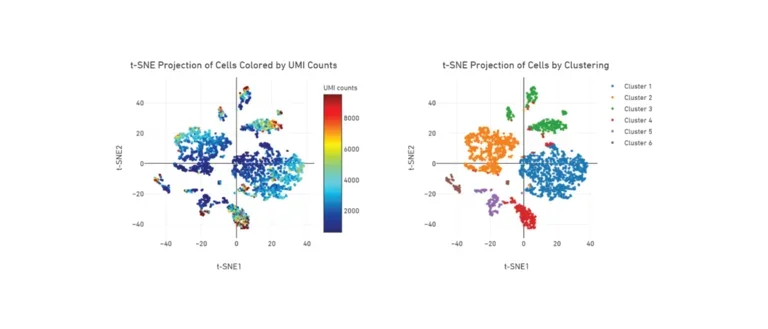
t-SNE Projection of Cells
t-SNE plot from single cell RNA sequencing of mouse nodose (vagal) ganglia showing heterogeneity of resident cell populations.
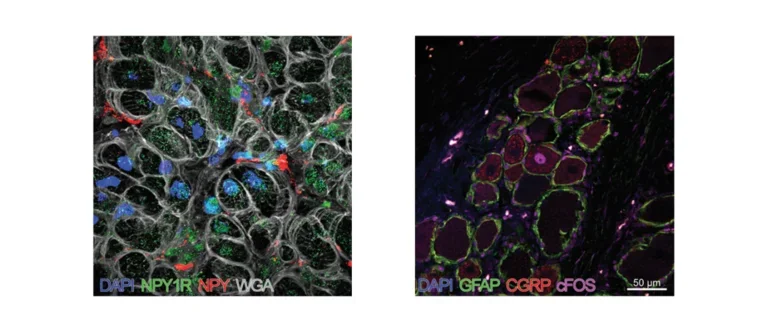
Stained Tissues
Left: Porcine ventricular myocardium stained for DAPI (cell nuclei), neuropeptide Y (NPY), NPY1-Receptor, and wheat germ agglutinin (WGA; plasma membranes). Right: Histology of dorsal root ganglion showing activation (cFos+) of pain sensory neurons (CGRP+) in the high thoracic spinal cord region, which provides innervation for the heart.
Researching Cardiac Autonomic Control of Heart Disease
Heart disease causes scar formation in the heart and pathological changes in the cardiac autonomic nervous system, which predispose to abnormal heart rhythms, including atrial and ventricular arrhythmias, and heart failure. The Vaseghi lab focuses on identifying the mechanisms and triggers for this neural remodeling and finding ways to prevent arrhythmias and reduce progression of heart failure by exploring a variety of neuromodulatory approaches. Our lab also has a special interest in better understanding differences in the presentation and mechanisms of arrhythmias and heart disease in men vs. women and finding therapies to improve women’s cardiovascular health.
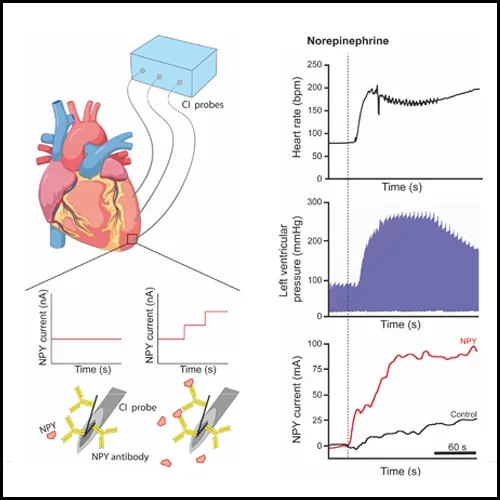
Tools and Techniques
- In-vivo Neural Recording of Peripheral Autonomic Ganglia
- Fast-Scanning Cyclic Voltammetry for Neurotransmitter Measurements
- Capacitive Immunoprobes for Neuropeptide Measurements
- High-resolution In-vivo Cardiac Electrophysiological Recordings and Mapping
- Optogenetics to Determine Role of Specific Neuronal Pathways
- Viral Tracing, Tissue Clearing, Immuno-histochemistry, Western Blotting, ELISA to Understand Tissue Innervation and Protein Expression
- Confocal and High-Resolution Microscopy
- Single Cell RNA-Sequencing
Announcements
- New paper entitled "Sympathovagal crosstalk: Y2-receptor blockade enhances vagal effects which in turn reduces NPY levels via muscarinic receptor activation" is accepted to Cardiovascular Research.
- Our paper entitled "Antiarrhythmic mechanisms of epidural blockade after myocardial infarction" is published in Circulation Research.
- 2 Abstracts accepted for Heart Rhythm 2025
- Our manuscript entitled "Myocardial infarction causes sex-dependent dysfunction in vagal sensory glutamatergic neurotransmission that is mitigated by 17β-estradiol" is published in JCI Insight
- 3 abstracts accepted for presentation at AHA Scientific Sessions 2024
- Dr. Valerie van Weperen wins Heart Rhythm Society's Young Investigator Award!

Meet the PI
Dr. Vaseghi is a cardiologist, clinical cardiac electrophysiologist, and physician scientist at the University of California, Los Angeles. She is a Professor of Medicine at UCLA and currently serves as the Vice Chair of the Research Committee of Heart Rhythm Society. She is the recipient of the NIH New Innovator Award. She obtained her MD from Stanford University and her PhD in Molecular, Cellular, and Integrative Physiology at UCLA. She is the principle investigator and co-investigator of several National Institute of Health funded studies evaluating the role of the autonomic nervous system in heart rhythm disorders and heart failure with a special interest in development of novel neuromodulatory therapies as well as catheter ablation techniques. Her laboratory’s breadth of work ranges from small and large animal models to human mechanistic studies to evaluate cardiac autonomic innervation, mechanisms of arrhythmias and heart failure, and sex differences underlying the presentation of cardiovascular disease.