Research
Our Mission
Cardiovascular disease is an important cause of morbidity and mortality in the U.S. and worldwide. Nearly all cardiovascular diseases are associated with cardiac autonomic nervous system dysfunction, which can initiate and cause further exacerbation of heart disease and abnormal heart rhythms. Many successful therapies for arrhythmias and heart failure have, in fact, targeted the cardiac autonomic nervous system. Significant changes occur in this nervous system with aging as well as with menopause that further predispose to heart rhythm disorders and heart failure. Our laboratory’s mission is to better understand the mechanisms behind these changes so that we can develop more personalized therapies for men and women.
Pictured: Immunofluorescence and confocal microscopy reveals respiratory neurons in green and cardiac neurons in red.
Neurocardiac Interactions
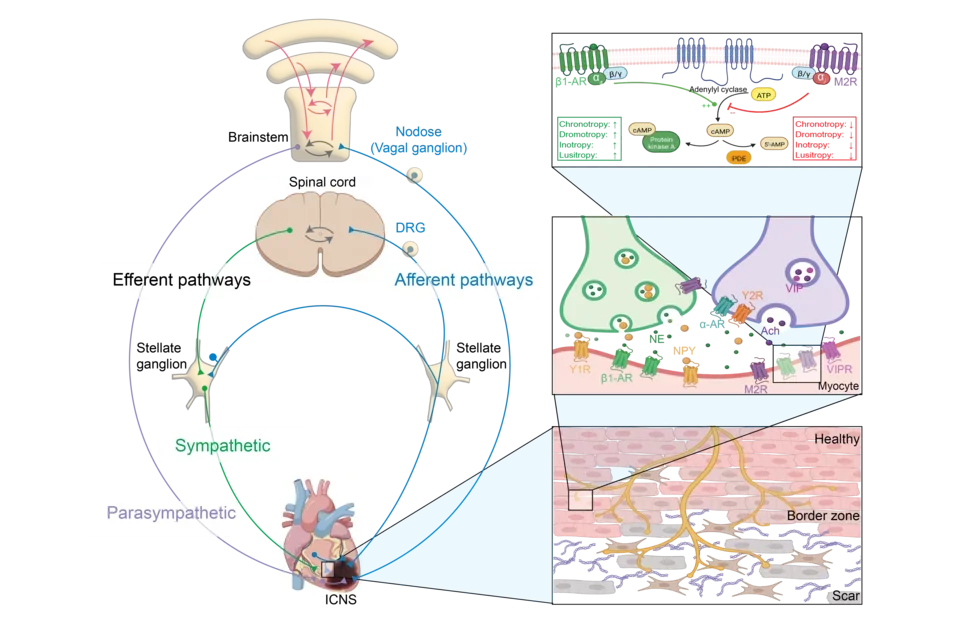
The heart is innervated by the sympathetic and parasympathetic branches of the nervous system. It is also continuously sending signals to the peripheral and central nervous system centers to modulate its function and meet physiological demand. In normal hearts, the sympathetic and parasympatehtic branches of the nervous system exist in a healthy balance to coordinate cardiovascular function on a beat-to-beat basis and to modulate cardiovascular parameters based on the needs of the body. In the setting of cardiovascular disease and aging, however, this autonomic balance undergoes pathological remodeling, characterized by sympathetic hyperactivity and parasympathetic withdrawal. Such autonomic imbalance can predispose to atrial and ventricular arrhythmias, lead to progression of heart failure, and increase the risk of sudden cardiac death. In addition, with onset of menopause, changes occur in this nervous system that further predispose to arrhythmias and heart failure.
Additional images depict:
- Mechanisms of neurocardiac interactions. The sympathetic and parasympathetic branches of the autonomic nervous system relay information either to the brain or directly back to the heart via intrinsic cardiac pathways, intrathoracic pathways, or centrally projecting pathways. These same pathways alter cardiac function by releasing neurotransmitters directly onto the heart.
- Sympathetic and parasympathetic neurons release neurotransmitters which bind to receptors on the heart to modulate various cardiovascular parameters, including heart rate, blood pressure, contractility, and speed of electrical conduction.
- Scar formation induced by myocardial infarction or other cardiovascular disease results in heterogenous innervation of scar and border-zone regions, which predisposes to ventricular arrhythmias.
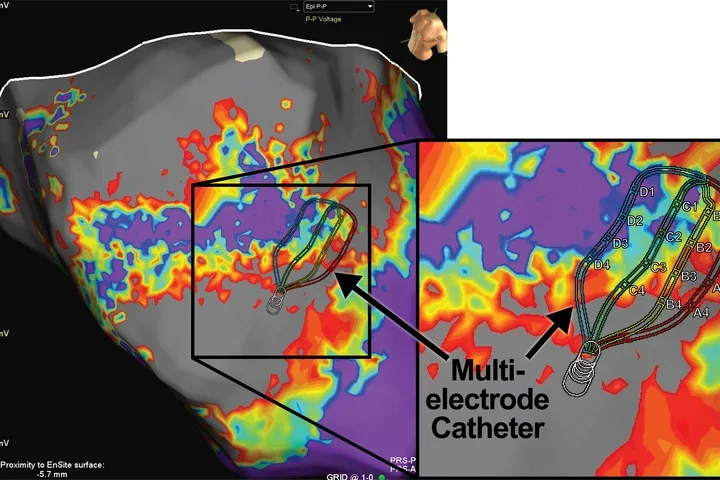
Techniques and Projects
We use advanced techniques such as viral tracing, single cell RNA sequencing, and optogenetics to better understand how cardiac innervation affects cardiac function and how molecular changes in this innervation predispose to heart failure and arrhythmias. Our laboratory has several ongoing studies on identifying the molecular markers of different subpopulations of cardiac neurons and support cells and examining how changes in these cells can contribute to arrhythmogenesis and progression of heart failure. By using optogenetics to activate specific neuronal pathways, we gain insight into the functional role of specific neuronal subpopulations in health and disease. Furthermore, these projects and techniques will allow us to study the effects of aging and sex, including hormonal changes with menopause, on the heart and its nervous system.
Leveraging the mechanistic insights obtained from small animal models, we can further validate the role of specific pathways and molecular changes in a translational large animal model to evaluate treatment options. These large animal studies allow us to evaluate the efficacy of various neuromodulatory therapies on diseased hearts using high-resolution electroanatomic mapping and electrophysiological recordings, with results that are more directly translated to patients.
Finally, in our clinical studies, we work directly with patients to study the anti-arrhythmic effects of neuromodulatory approaches such as vagal nerve stimulation. Ultimately, the mission of our research is to translate our findings from the bench to the bedside, develop more personalized therapies for women and men, and help improve the lives of the millions of patients that live with heart disease.
Additional Graphics
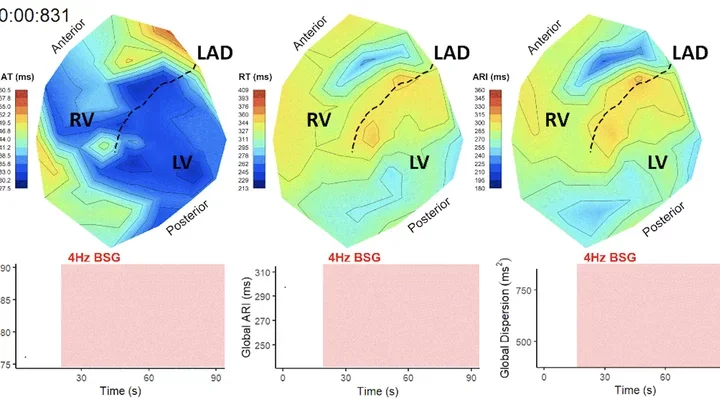
Pictured: Polar maps are spatial depictions of the duration of cardiac action potentials across both ventricles of the heart. In response to pathological changes caused by cardiovascular disease, as well as various interventions including drug administration and nerve stimulation, differences in action potential duration across the heart can be observed.
Pictured: MRI images of an infarcted heart display transverse cross-sections of each of the heart's chambers and an anteroseptal scar.